
NIH-funded clinical trial shows risk is similar whether or not a cloudy lens is replaced with a lens implant. Study suggests lifelong monitoring is crucial to preventing glaucoma-related vision loss.
Children who undergo cataract surgery as infants have a 22% risk of glaucoma 10 years later, whether or not they receive an intraocular lens implant. The findings come from the National Eye Institute (NEI)-funded Infant Aphakic Treatment Study, which today published 10-year follow-up results in JAMA Ophthalmology. NEI is part of the National Institutes of Health.
“These findings underscore the need for long-term glaucoma surveillance among infant cataract surgery patients. They also provide some measure of assurance that it is not necessary to place an intraocular lens at the time of cataract surgery,” said Michael F. Chiang, M.D., director of NEI.
 “The results challenge the notion that replacing the child’s lens with an implanted one protects the child from developing glaucoma, a belief among some pediatric ophthalmology surgeons,” said the trial’s principal investigator, Scott R. Lambert, M.D., professor of ophthalmology at Stanford University, Palo Alto, California.
“The results challenge the notion that replacing the child’s lens with an implanted one protects the child from developing glaucoma, a belief among some pediatric ophthalmology surgeons,” said the trial’s principal investigator, Scott R. Lambert, M.D., professor of ophthalmology at Stanford University, Palo Alto, California.
At the time of cataract removal, the 114 study participants (ages 1-6 months) had been born with cataract in one eye. In the operating room, the infants were randomly assigned to receive an artificial lens implant or go without a lens, a condition called aphakia.
Annually, fewer than 2,500 children in the U.S. are born with cataract, a clouding of the eye’s lens. Surgery is used to remove and replace the cloudy lens. To allow the child’s eye to focus light properly following removal of the cataract, an intraocular lens implant may be placed at surgery, or the eye may be left aphakic, and a contact lens (or glasses, if both eyes have had a cataract removed) may be used to provide the needed correction.
“I tell patients’ parents that implanting a lens in the infant’s eye is like buying your child’s wedding shoes when they’re an infant. It is hard to predict what final power the intraocular lens should have, without knowing how that eye will grow over the years, so placing a lens at the time of cataract removal in an infant involves estimation, and may not turn out to be correct. Hence the eye may end up needing strong glasses or even replacement of the original lens implant.,” said the lead author on the paper, Sharon F. Freedman, M.D., a pediatric glaucoma specialist at Duke University, Durham, North Carolina.
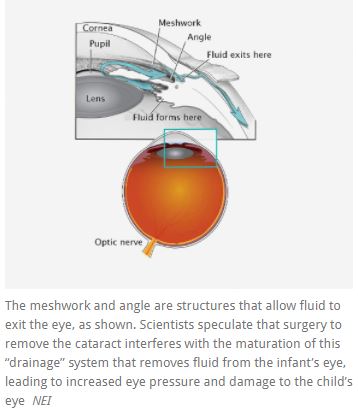
Children who undergo cataract removal have an increased risk of glaucoma, a sight-threatening condition that damages the optic nerve—the connection between the eye and brain. Scientists speculate that surgery to remove the cataract interferes with the maturation of how fluid flows out of the infant’s eye leading to increased eye pressure and optic nerve damage in some of these eyes.
Among the 110 children who were available for re-examination at 10 years, 25 eyes (24%) had developed glaucoma, and 21 eyes (20%) were glaucoma suspects due to elevated eye pressure. However, visual acuity was similar among those eyes that developed glaucoma compared to those eyes that had not. The researchers found no evidence of glaucoma-related eye damage, assessed by imaging of the optic nerve head to measure the retinal nerve fiber layer thickness.
The investigators attribute the absence of glaucoma-related eye damage to close patient monitoring, as any sign of glaucoma was aggressively treated.
While the lifetime glaucoma risk trajectory for patients who have cataract removal as infants remains unknown, this study found that the risk of glaucoma after cataract removal rose from 9% at 1 year, to 17% at 5 years, and to 22% at 10 years.
“Any child who has had a cataract removed needs to be seen by an eye care provider once a year at a minimum,” said Freedman. “Any child diagnosed with glaucoma or above-normal intraocular pressure without signs of ocular damage — what we called glaucoma suspect — should be monitored every four to six months depending upon the stability of the condition and the health of the eye.”
At 10 years, 40% of the followed children had developed the diagnosis of glaucoma or glaucoma suspect. A glaucoma suspect is an eye that has above normal eye pressure or another feature suspicious but not diagnostic of glaucoma.
The findings also confirm that the timing of cataract surgery is a balancing act: Whereas surgery at younger ages increases glaucoma risk, delaying surgery increases risk of amblyopia, a leading cause of visual impairment in children that results when cataract in one eye causes the brain to ignore signals from that eye and favor the other eye.
Future studies of glaucoma following cataract surgery in children will benefit from groundwork by the Infant Aphakic Treatment Study. Freedman said collaboration among the 12 study centers defined diagnostic standards for pediatric glaucoma and glaucoma suspect and criteria for glaucoma-related adverse events. “This cohort began the process leading to an international classification of childhood glaucoma in 2013 that is used around the world today,” she said.
Investigators at Emory University, Harvard University, Duke University, Indiana University, Vanderbilt University, Medical University of South Carolina, University of Minnesota, Cleveland Clinic, Baylor University, Oregon Health and Science University, Miami Children’s Hospital, and University of Texas Southwestern helped lead the trial. NEI funding includes grants UG1EY013287, UG1EY013272, UG1EY025553, and P30EY026877. The clinicaltrial.gov identifier is NCT00212134.
NEI leads the federal government’s research on the visual system and eye diseases. NEI supports basic and clinical science programs to develop sight-saving treatments and address special needs of people with vision loss. For more information, visit https://www.nei.nih.gov.
About the National Institutes of Health (NIH): NIH, the nation's medical research agency, includes 27 Institutes and Centers and is a component of the U.S. Department of Health and Human Services. NIH is the primary federal agency conducting and supporting basic, clinical, and translational medical research, and is investigating the causes, treatments, and cures for both common and rare diseases. For more information about NIH and its programs, visit www.nih.gov.
NIH…Turning Discovery Into Health®
References
Freedman, SF; Beck, AD; Nizam, A; Vanderveen, DK; Plager, DA; Morrison, DG; Drews-Botsch, CD; Lambert, SR; The Infant Aphakia Treatment Study Group. “Glaucoma-related adverse events at 10 years in the Infant Aphakia Treatment Study: a randomized clinical trial”. Published December 17, 2020, Journal of the American Medical Association Ophthalmology.
News release originally appeared in NIH News