This appeared on WRAL on January 19, 2017
DURHAM, N.C. — A new treatment to stop a progressive eye disease was approved recently by the Food and Drug Administration and could save patients from more drastic options in the future.
The disease, Keratoconus, is a progressive and possibly genetic disease of the cornea that damages a person's vision. Duke University's Eye Center is working to save patients' eyesight as the only provider of the new procedure in North Carolina.
Madison Kroger, 20, of Charlotte first noticed problems in her left eye about six years ago when her vision started to get blurry. Initially, her optometrist thought it was just nearsightedness.
Later, though, Kroger learned she had Keratoconus.
"(Keratoconus is) where the cornea undergoes an abnormal shape because of the weakening of its structure," said Duke Opthalmologist Dr. Terry Kim.
"The treatment has been glasses and contact lenses, but when Keratoconus gets worse, unfortunately, the vision cannot be corrected," Kim said.
Kim says the last option is a corneal transplant.
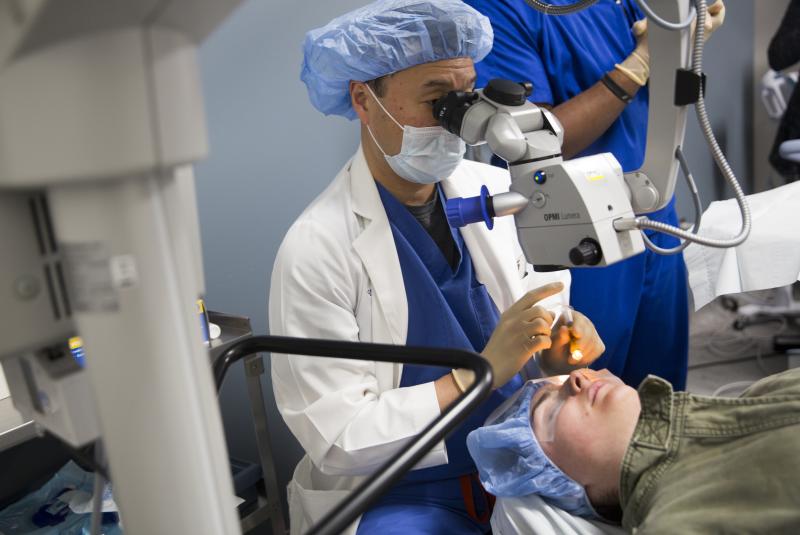
To avoid a transplant, Kroger is getting the newly approved treatment, called corneal cross-linking, or CXL.
Kroger is Duke Eye Center's first patient to get the treatment.
"It is exciting that I'm the first person to have it done," Kroger said. "It's not like it's never been seen before. It's just new here, which is exciting."
During the procedure, Kim will remove the surface layer of the cornea and place drops of riboflavin—or vitamin B-2—on Kroger's eye.
"We really want that riboflavin to penetrate into the cornea," Kim said.
About 30 minutes later, ultra-violet light helps the cornea absorb the vitamins and produce chemical bonds across its collagen layers.
The goal is to prevent further changes in the shape.
The best effects are in younger patients, like Kroger, who had an early diagnosis.
"But in the future, when I get hard contact lenses, they should work a lot better," Kroger said.
Because CXL is new, insurance does not cover the costs of the procedure. Kim said the procedure has been performed for several years outside the U.S. and is proven safe and effective.