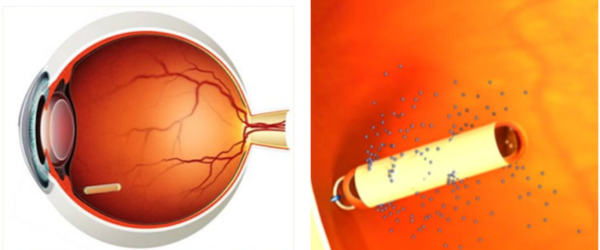
An important milestone has been reached for patients with idiopathic macular telangiectasia type 2 (MacTel), with the FDA approval of revakinagene taroretcel-lwey or ENCELTO, the first and only treatment for MacTel.
This treatment utilizes an encapsulated cell therapy technology designed to continually deliver ciliary neurotrophic factor to the retina to slow the progression of the disease. Results from the two phase 3 registration trials showed that this surgical implant significantly decreased the loss of macular photoreceptors in MacTel patients over 24 months. The treatment is expected to be available in the United States for patients starting in June 2025.
MacTel is usually bilateral and diagnosed in middle-aged adults. It’s characterized by the growth of leaky, damaging blood vessels as well as alterations to the retinal vasculature network. Risk factors for MacTel include hypertension and diabetes. MacTel can also run in families; the condition may have genetic risk factors.
The Duke Reading Center, led by Director Glenn Jaffe, MD along with Duke researchers, including Sina Farsiu, PhD, Eleonora Lad, MD, PhD, and Sandra Stinnett, PhD, performed the data analyses for the registration trials and was central to the FDA approval. These trials also pioneered the use of a novel clinical trial endpoint, ellipsoid zone (photoreceptor) loss area, which is already being employed in clinical trials of other retinal diseases.