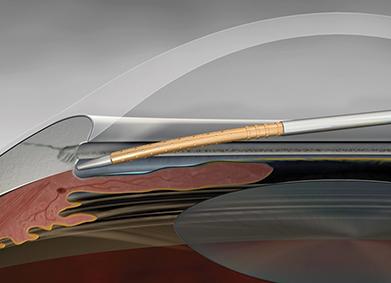
Duke Eye Center glaucoma surgeons are now offering Alcon's recently FDA-approved Cypass glaucoma micro-shunt implant. A minimally invasive glaucoma surgery (MIGS), that offers a lower surgical risk and an alternative to lifelong suffering from use of glaucoma drops. Implanted at the time of cataract surgery, the Cypass micro-shunt will help maintain current levels of vision and prevent further deterioration.
A significant level of confusion, anxiety, and frustration is associated with glaucoma eye drop use. Patients struggle to keep up with monthly refills due to the cost of the medications and the requirement to use the drops multiple times a day with several different drops is also challenging.
Typically, glaucoma patients would need to fail use of 3-4 different glaucoma eye drops before we start to consider glaucoma surgery as a treatment option. With eye drops, patients inevitably suffer from chronic redness, constant irritation, mucus, dry eyes, blurry vision, eyelid scarring, and allergies.
Traditional glaucoma surgeries – even when performed successfully -- carry high risks of vision loss in the operating room and beyond. These surgeries involve extensive incisions and implants, and have a relatively long recovery period when compared to cataract surgery. Patients have a lifelong risk of increased eye infections and often still need multiple eye drops despite these surgeries. As such, glaucoma surgeries are usually reserved for patients at a much later stage in the treatment plan, or for patients who present with very severe disease.
The new CyPass implant involves minimal surgical manipulation and is entirely contained within the eye. Studies performed for FDA-approval showed much lower surgical risks and complications, thus allowing earlier use of this implant. In particular, this implant is especially useful in patients who suffer eye drop side-effects.
This new implant can accomplish treatment goals with fewer surgical risks and thus glaucoma specialists can now include surgery as a treatment option much earlier and for patients with mild glaucoma. It would help reduce financial costs, reduce compliance issues and debilitating chronic side-effects from eye drops.