
The side-effect profile is significant, but a few approaches may help to extend medical treatment in selected cases.


Surgical retinal vein cannulation during vitrectomy surgery in eyes with CRVO has also been explored with promising improvements in visual acuity.9 Because histopathologic studies have demonstrated a thrombus at the level of the lamina cribrosa in eyes with CRVO, targeted retinal venous cannulation can allow direct access to the thrombus, which may be displaced with injection of balanced salt solution alone or dissolved with recombinant tissue plasminogen activator, ocriplasmin (Jetrea, Oxurion) or other thrombolytic.
Animal studies are evaluating retinal cannulation for the treatment of RVO, both unassisted and with robotic assistance for more distal, difficult-to-access vasculature.10,11 Recently, a report of four RVO patients undergoing robot-assisted retinal vein cannulation was published, showing both technical feasibility and a reasonable safety profile.12
Vitrectomy with and without ILM peeling 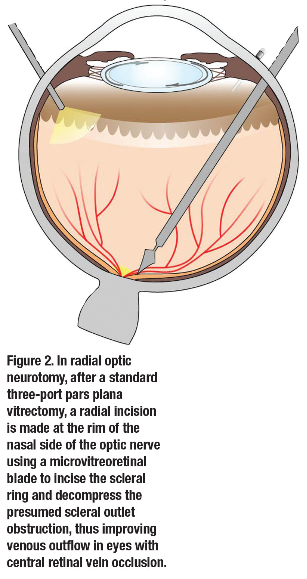
Prior studies have evaluated the role of vitrectomy with removal of the posterior hyaloid with and without internal limiting membrane peeling to treat macular
edema associated with RVO, particularly when the edema is refractory to treatment with intravitreal
anti-VEGF or corticosteroid therapy.
Multiple studies have shown decreased retinal thickness and improved visual acuity in eyes with RVO after PPV with removal of the posterior hyaloid alone, without ILM peeling.13-15 Prior studies have suggested that the vitreous may be a reservoir for inflammatory and growth factor mediators, including interleukin-6 and vascular endothelial growth factor.16-18 Therefore, removal of the vitreous alone may lead to resolution of macular edema on a cellular level with reduced retinal hypoxia, decreased vascular permeability, and improved oxygenation of the inner retina as more oxygenated aqueous fills the vitrectomized vitreous cavity.19
In one study, eyes with higher preoperative intravitreal anti-VEGF levels had less visual acuity gains after PPV.15 This suggests that there may be a correlation and the potential to predict which eyes with RVO will be most responsive to surgical management in the future.
While ILM peeling is thought to primarily relieve mechanical forces contributing to macular edema, such as tangential traction, the added step of ILM peeling may provide the extra benefit of decompression of the inner retina.20 Furthermore, removal of the ILM may also possibly decrease macular edema by stimulating a neural repair process.21 While ILM peeling may confer anatomic benefits, whether or it provides a functional benefit is still up for debate.
The largest study of this approach, the European VitreoRetinal Society Macular Edema Study, evaluated visual acuity outcomes in a nonrandomized fashion at 24 months postoperatively following PPV with ILM peeling vs. treatment with intravitreal anti-VEGF or steroid injections. In more than 700 cases of CRVO or BRVO, the study reported significantly greater visual gains in eyes treated with surgical management.22
On the other hand, smaller retrospective case series have reported a decrease in retinal thickness without significant visual acuity gains in eyes with CRVO after PPV with ILM peeling.23,24 These studies have shown heterogeneity in the time duration from development of RVO to surgery, extent of retinal ischemia, lens status and surgical technique. No randomized trial has evaluated the utility of PPV with or without ILM peeling for the treatment of macular edema secondary to RVO.
Pitfalls and potential of vitrectomy surgery in eyes with RVO
Reperfusion of the retina continues to be a primary goal in vitreoretinal surgical techniques for management of RVO. However, no definitive studies have reproducibly shown the visual benefit of vitrectomy with either AV sheathotomy, radial optic neurotomy, creation of a chorioretinal anastomosis or retinal vein cannulation thus far. Furthermore, the side-effect profile for all of these techniques includes vision-threatening complications, including choroidovitreal neovascularization, which may persist despite aggressive treatment in some eyes.
With the introduction of anti-VEGF treatment and corticosteroid options, these more invasive surgical options have become less common in the armamentarium of treatment options for eyes  with retinal vein occlusion.
with retinal vein occlusion.
However, PPV, removal of the posterior hyaloid and possible ILM peeling may derive added benefit to existing treatments, particularly in cases of persistent macular edema that would otherwise require frequent intravitreal treatment. The half-life of intravitreal anti-VEGF medications in vitrectomized eyes is shorter than in nonvitrectomized eyes, so they clear faster and have decreased efficacy.25 Thus, longer- acting adjuvant treatment, such as more durable anti-VEGF agents or corticosteroids, may need to be considered after PPV in eyes with RVO.
Bottom line
The known anatomical improvement from PPV with or without ILM peeling should be balanced with the likelihood of associated visual improvement. We should consider this in the context of several other factors, such as retinal perfusion status, the length of time since the initial event and concurrent ophthalmic comorbidities that may limit visual acuity. RS
Article originally appeared in Retina Specialist