Overview
The biology and pathophysiology of vertebrate photoreceptor cells
Rod and cone photoreceptors are sensory neurons responsible for the detection of light and transforming the information entering the eye in the form of photons into neuronal activity ultimately transmitted to our brain. Our laboratory is interested in elucidating the molecular mechanisms underlying signal transduction, subcellular compartmentalization and maintenance of the healthy state of these cells.
Support Inherited Retinal Disease Discovery
Research Projects
Currently, we pursue three major experimental directions:
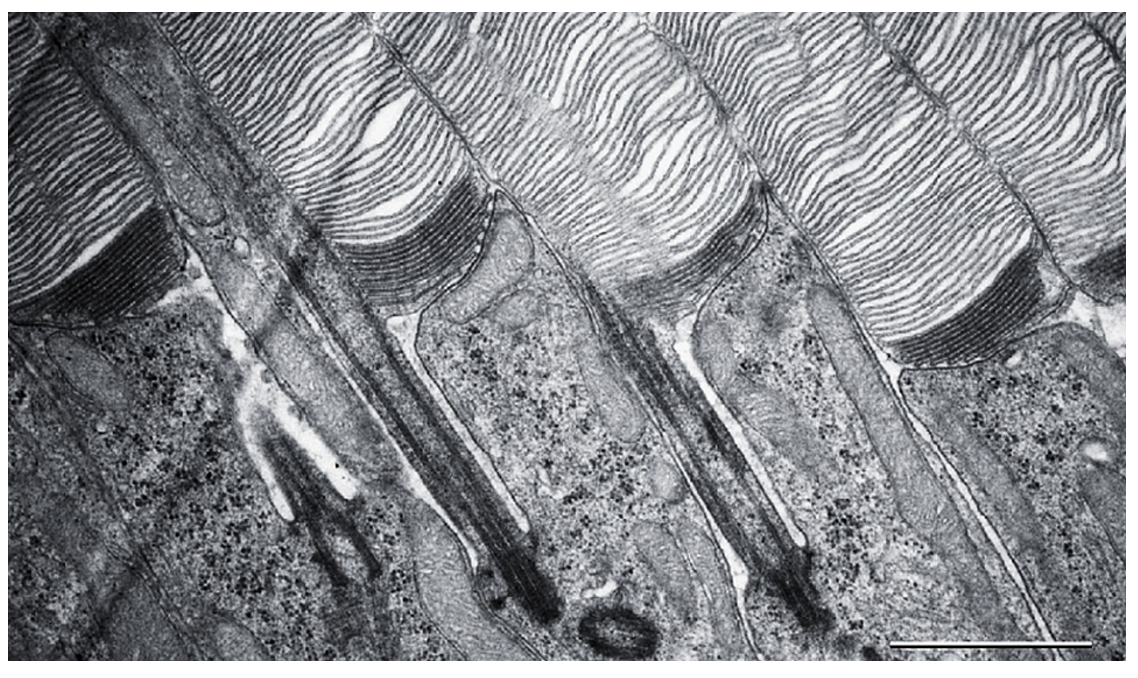
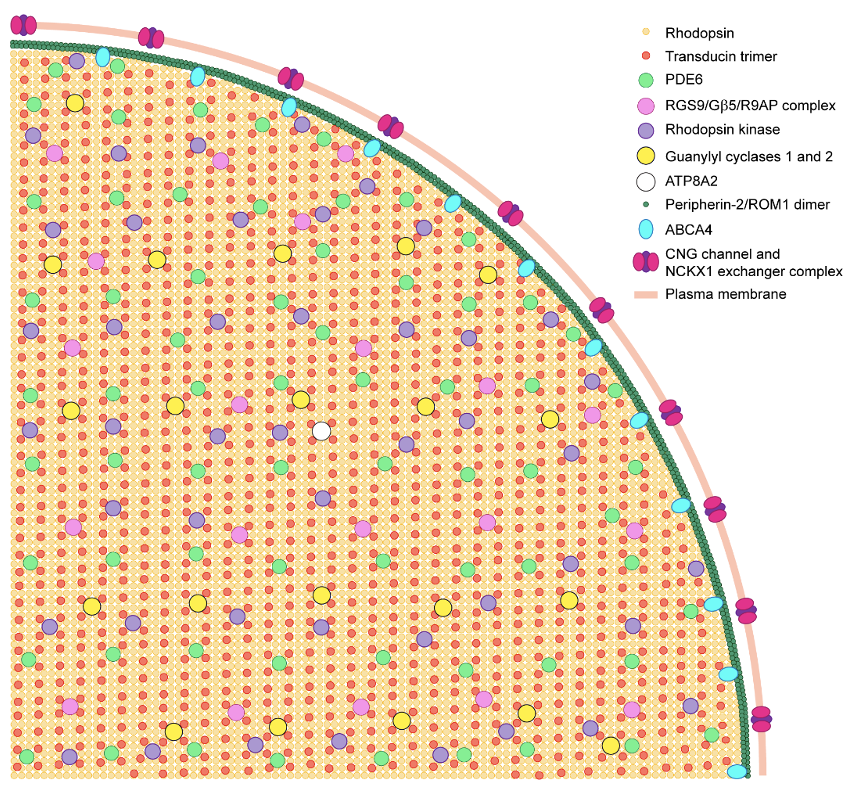
Photoreceptors are highly compartmentalized neurons, with all molecular events responsible for generating light-signals confined to the outer segment, a ciliary organelle containing a stack of flattened membranous “discs”. Building this organelle involves two interconnected processes – formation of its unique anatomical structure and populating it with a unique set of signaling and structural proteins. We study both of these processes in our laboratory.
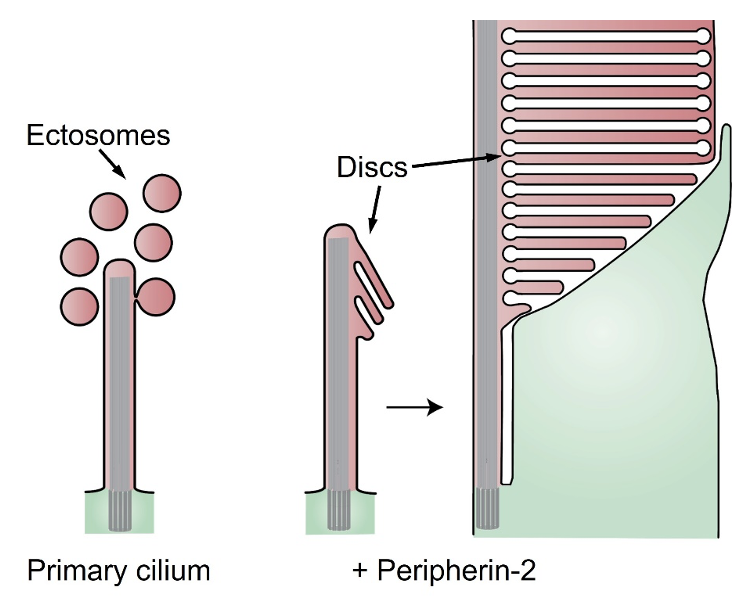
Central to our interests is the complex process of photoreceptor disc morphogenesis, which continues throughout the entire lifetime of a photoreceptor. A new disc is added at the base of the outer segment every ~20 minutes, while old discs are shed from the outer segment tip by the retinal pigment epithelium. The formation of a new disc starts with evagination of the plasma membrane at the outer segment base, followed by lateral membrane outgrowth, flattening, and, in the cases of rods and mammalian cones, subsequent disc enclosure. Our laboratory investigates every step of this process. Several years ago, we made an important conceptual connection between the processes of photoreceptor disc morphogenesis and the formation of extracellular vesicles, ectosomes, ubiquitously produced by various primary cilia. We demonstrated that, like most other primary cilia, the photoreceptor outer segment has an innate ability to release ectosomes, but in normal photoreceptors this process is suppressed by the protein called peripherin-2 (akaPRPH2 or rds). Membranes retained at the outer segment through this mechanism are used to build photoreceptor discs through their elongation, flattening and enclosure.
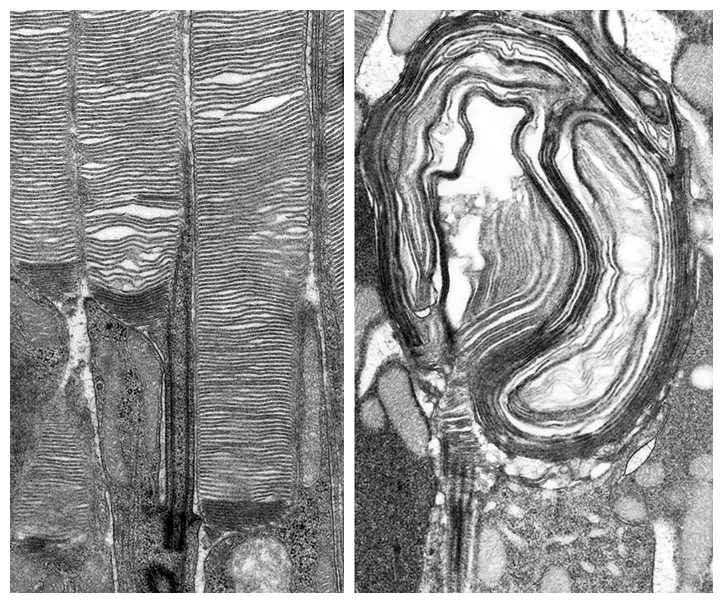
A critical role in disc morphogenesis is played by actin cytoskeleton whose expansion drives membrane evagination upon formation of new discs. We found that this process is dependent on the polymerization of branched actin in a mechanism resembling the formation of lamellipodia in migrating cells. This actin network expansion is initiated by the pentameric WAVE complex containing a unique subunit, WASF3. WAVE complexes are known to mediate between the upstream signaling pathways and downstream actin networks. Thus, our current efforts are directed to identifying such an upstream signaling pathway that is responsible for initiating the formation of each new disc with the striking frequency of approximately 80 times per day.
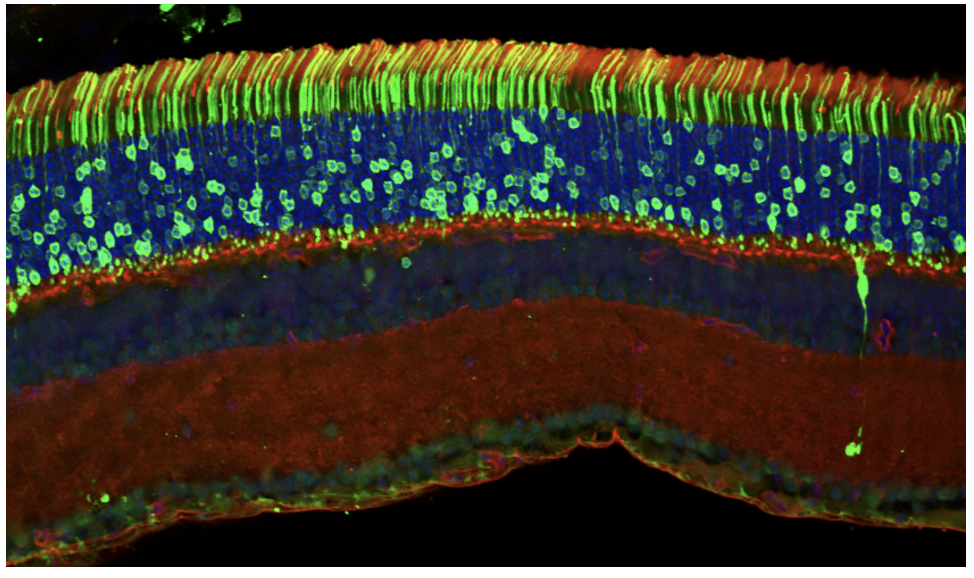
Our second major direction is to address the pathobiological mechanisms underlying degenerative diseases of the retina and develop practical approaches to ameliorate these debilitating conditions. Previously, we showed that a major stress factor leading to photoreceptor cell death in a broad spectrum of inherited retinal degenerations is “proteasomal overload,” i.e., insufficient capacity of the ubiquitin-proteasome system to degrade vast amounts of misfolded or mistargeted proteins produced as a result of the underlying mutations. We further demonstrated that genetic enhancement of proteasomal activity in mouse rods results in a striking delay in photoreceptor degeneration resulting from mutations in multiple proteins, including the relatively common P23H mutation of rhodopsin. Currently, we are focused on exploring translational implications of these studies by seeking practical approaches to enhance photoreceptor proteostasis using small molecules and gene therapy approaches.
In the course of our studies, we are exploring high-end applications of mass spectrometry-based proteomics. Our laboratory was the first to adopt several advanced proteomic approaches to our field. One of them is protein correlation profiling used for elucidation of multiple protein distributions among different compartments of the photoreceptor cells and for identification of unique protein components of various photoreceptor membranes. Using this approach, we demonstrated that a small protein PRCD (progressive rod and cone degeneration) is a unique component of photoreceptor discs and subsequently identified three novel unique components of the plasma membrane enclosing the rod outer segment. Most recently, we adopted a highly efficient and accurate methodology for simultaneous absolute quantification of many proteins in the same sample, termed “MS Western”. This method allowed us to determine the precise molar ratio amongst all major functional and structural proteins residing in the light-sensitive outer segments of photoreceptor cells.
Lab Members

Left to right front row: Natasha Klementeva, PhD, Lauren Cao, BS, Lin Yu, PhD, Stella Finkelstein, MS, Carson Castillo, MS, Margaux Kreitman, PhD, Vadim Arshavsky, PhD
- Kirill Martemyanov, Ph.D. Professor and Chair, Department of Neuroscience, Scripps Institute, Florida
- Chuck Dumke, Ph.D. Professor of Health and Human Performance, University of Montana
- Polina Lishko, Ph.D. Professor of Cell Biology and Physiology, Washington University
- Peter Calvert, Ph.D. Professor of Ophthalmology, SUNY Upstate Medical University
- Maxim Sokolov, Ph.D. Professor of Neuroscience, West Virginia University
- Marina Antoch, Ph.D. Professor, Roswell Park Cancer Institute
- Sheila Baker, Ph.D. Associate Professor of Biochemistry, University of Iowa
- Mickey Kosloff, Ph.D. Associate Professor of Human Biology, Haifa University
- Ekaterina Lobanova, Ph.D. Associate Professor of Ophthalmology, University of Pittsburgh
- Sidney Gospe, MD., PhD Assistant Professor of Ophthalmology, Duke University
- Jillian Pearring, Ph.D. Assistant Professor of Ophthalmology, University of Michigan
- Raquel Salinas, Ph.D. Associate Member, MD Anderson Cancer Center
- William Spencer, Ph.D. Assistant Professor of Ophthalmology, SUNY Upstate Medical University
- Ilya Leskov, M.D., Ph.D. Assistant Professor of Ophthalmology, SUNY Downstate Health Sciences University
- Boris Riedel, Ph.D. Assistant Professor of Pathology, University of North Carolina
- Katherine Strissel, Ph.D. Scientist, Boston University
- Richard Rustandi, Ph.D. Research Fellow, Merck
- Elina Makino, Ph.D. Scientific Advisor, biotech/venture financial groups
- Rolf Herrmann, Ph.D. Director of Translational Medicine & Early Asset Lead, Boehringer Ingelheim
- Arye Elfenbein, M.D., Ph.D. Co-founder, Wildtype
Selected Publications
Skiba, N.P., Lewis. T.R., Spencer, W.J., Castillo, C.M., Shevchenko, A., Arshavsky, V.Y. Absolute quantification of photoreceptor outer segment proteins. J. Proteome Res. (2023) 22, 2703-2713.
Skiba, N.P., Cady, M.A., Molday, L., Han, J.Y.S., Lewis, T.R., Spencer, W.J., Thompson, W.J., Hiles, S., Philp, N.J., Molday, R.S., Arshavsky, V.Y. TMEM67, TMEM237 and embigin in the complex with lactate transporter MCT1 are unique components of the photoreceptor outer segment plasma membrane. Mol. Cell. Proteomics (2021) 20:100088.
Spencer, W.J., Lewis, T.R., Phan, S., Cady, M.A., Serebrovskaya, E.O., Schneider, N.F., Kim, K.-W., Cameron, L.A., Skiba, N.P., Ellisman, M.H., Arshavsky, V.Y. Photoreceptor disc membranes are formed through an Arp2/3-dependent lamellipodium-like mechanism. Proc. Natl. Acad. Sci. USA. (2019) 116, 27043-27052.
Lobanova, E.S., Finkelstein, S., Li, J., Travis, A.M., Hao, Y., Klingeborn, M., Skiba, N.P., Deshaies, R.J., Arshavsky, V.Y. Increased proteasomal activity supports photoreceptor survival in inherited retinal degeneration. Nat. Commun. (2018) 9:1738.
Salinas, R.Y., Pearring, J.N., Ding, J.D., Spencer, W.J., Hao, Y., Arshavsky, V.Y. Photoreceptor discs form through peripherin-dependent suppression of ciliary ectosome release. J. Cell Biol. (2017) 216, 1489-1499.
Positions Available
Our lab is currently looking to fill a postdoctoral position to study basic and translational photoreceptor biology.
Interested candidates, please send your CV via email to Vadim Arshavsky.





